
国际生殖健康/计划生育 ›› 2021, Vol. 40 ›› Issue (5): 391-396.doi: 10.12280/gjszjk.20210121
收稿日期:2021-03-17
出版日期:2021-09-15
发布日期:2021-09-29
通讯作者:
符淳
E-mail:fuchun0814@csu.edu.cn
基金资助:
ZHOU Zhi-xian, ZHU Fang, YIN Huan, SU Ye, CAI Hai-yi, FU Chun( )
)
Received:2021-03-17
Published:2021-09-15
Online:2021-09-29
Contact:
FU Chun
E-mail:fuchun0814@csu.edu.cn
摘要:
在哺乳动物卵巢储备的形成过程中,生殖细胞处于DNA复制、交换和重组的活跃期,对各种内外损伤因素敏感,易发生DNA损伤。多条DNA损伤修复途径在此期间发挥作用。同源重组途径修复DNA双键断裂,保护和重启停滞复制叉;范可尼贫血途径修复链间交联,促进复制叉重启;碱基切除修复途径是全基因组的表观遗传编程的重要机制;错配修复途径维持减数分裂进程,促进重组过程的交叉形成和稳定;核苷酸切除修复途径促进修复链间交联等。这些功能对于维持生殖细胞基因组稳定、减少生殖细胞凋亡、促进生殖细胞增殖和多能性表达以调节原始生殖细胞发育形成卵巢储备至关重要。更好了解哺乳动物卵巢储备形成中的DNA损伤修复,为原发性卵巢功能不全的病因学解析提供理论基础。
周志贤, 朱芳, 殷缓, 苏叶, 蔡海奕, 符淳. 哺乳动物卵巢储备形成中的DNA损伤修复[J]. 国际生殖健康/计划生育, 2021, 40(5): 391-396.
ZHOU Zhi-xian, ZHU Fang, YIN Huan, SU Ye, CAI Hai-yi, FU Chun. DNA Damage and Repair during Ovarian Reserve Formation[J]. Journal of International Reproductive Health/Family Planning, 2021, 40(5): 391-396.
| 修复途径 | 作用阶段 | 损伤因素 | 损伤类型 | 修复作用 |
|---|---|---|---|---|
| BER途径 | 表观遗传修饰 | 内源烷基化剂、内源代谢物、辐射 | 小DNA损伤和加合物 | 替换单个受损核苷酸 |
| MMR途径 | DNA复制、减数分裂重组 | 自发性dNTP错配、辐射 | 碱基错配、插入、缺失 | 修复dNTP错误匹配 |
| HR途径 | 有丝分裂、减数分裂重组 | 减数分裂重组诱导、辐射 | DSB、停滞复制叉 | 修复DSB、恢复停滞复制叉 |
| FA途径 | 有丝分裂、减数分裂重组 | 内源代谢物、链间交联剂、辐射 | ICL、停滞复制叉 | 修复ICL、协调多种DNA修复活动 |
| NER途径 | DNA交联 | DNA交联、辐射 | 大块DNA加合物、严重DNA损伤 | 更换大段DNA |
表1 原始卵泡形成过程中的DNA损伤修复途径
| 修复途径 | 作用阶段 | 损伤因素 | 损伤类型 | 修复作用 |
|---|---|---|---|---|
| BER途径 | 表观遗传修饰 | 内源烷基化剂、内源代谢物、辐射 | 小DNA损伤和加合物 | 替换单个受损核苷酸 |
| MMR途径 | DNA复制、减数分裂重组 | 自发性dNTP错配、辐射 | 碱基错配、插入、缺失 | 修复dNTP错误匹配 |
| HR途径 | 有丝分裂、减数分裂重组 | 减数分裂重组诱导、辐射 | DSB、停滞复制叉 | 修复DSB、恢复停滞复制叉 |
| FA途径 | 有丝分裂、减数分裂重组 | 内源代谢物、链间交联剂、辐射 | ICL、停滞复制叉 | 修复ICL、协调多种DNA修复活动 |
| NER途径 | DNA交联 | DNA交联、辐射 | 大块DNA加合物、严重DNA损伤 | 更换大段DNA |

图3 HR途径修复DSB,保护和重启复制叉 注:a. HR修复DSB成CO或NCO。减数分裂重组过程中,DSB被SPO11诱导形成。MRN复合物定位于DNA保持断端连接。在酶作用下产生3′端单链DNA尾巴被复制蛋白A(replication protein A,RPA)包裹。在BRCA2的协助下,RAD51和DMC1取代RPA并协助损伤的DNA入侵同源模板链。由此DSB修复,在合成依赖链复性(synthesis-dependent strand annealing,SDSA)路径中形成NCO;在另一路径中双霍尼迪结(dHJ)形成,随后发生不对称裂解,最终形成CO。b. HR和FA途径保护复制叉。DNA损伤使复制叉停滞。BRCA1、BRCA2和FANCD2蛋白稳定RAD51以保护停滞复制叉和新生链,阻止核酸酶MRE11对新生链的降解作用。c. HR重启停滞复制叉。(i)HR途径修复单侧DSB或主导链间隙,解决主导链停滞。(ii)HR途径修复滞后链间隙,解决滞后链停滞。最终复制叉重启。
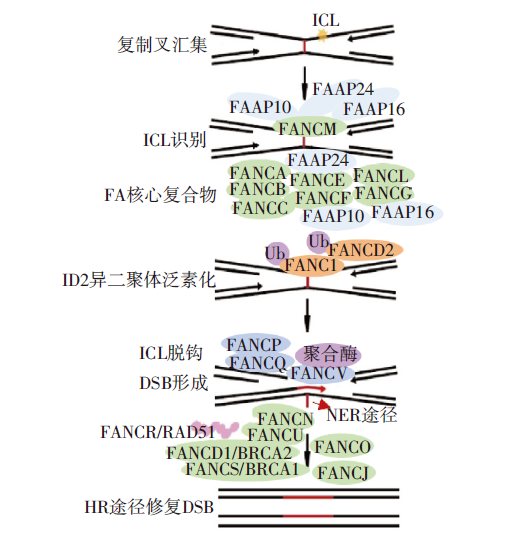
图4 FA途径修复ICL 注:两个复制叉在ICL位点汇集并停滞。锚复合物(FANCM-FAAP24、10、16)识别定位于ICL。FA核心复合物(FANCA、B、C、E、F、G、L和FAAP20、100)被募集,随后催化FANCI-FANCD2异二聚体的泛素化激活过程。FANCI-FANCD2作为平台招募其他FA蛋白。FANCP和FANCQ催化ICL脱钩。FANCV组成的多亚基聚合酶绕过DNA损伤实现跨病变DNA合成。NER途径去除脱钩后残余加合物。最后,ICL修复产生的DSB由HR修复。
| [1] |
Lew R. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure[J]. Best Pract Res Clin Obstet Gynaecol, 2019, 55:2-13. doi: 10.1016/j.bpobgyn.2018.05.005.
doi: 10.1016/j.bpobgyn.2018.05.005 URL |
| [2] |
Huhtaniemi I, Hovatta O, La Marca A, et al. Advances in the Molecular Pathophysiology, Genetics, and Treatment of Primary Ovarian Insufficiency[J]. Trends Endocrinol Metab, 2018, 29(6):400-419. doi: 10.1016/j.tem.2018.03.010.
doi: 10.1016/j.tem.2018.03.010 URL |
| [3] |
Patel H, Bhartiya D, Parte S. Further characterization of adult sheep ovarian stem cells and their involvement in neo-oogenesis and follicle assembly[J]. J Ovarian Res, 2018, 11(1):3. doi: 10.1186/s13048-017-0377-5.
doi: 10.1186/s13048-017-0377-5 URL |
| [4] |
Stringer JM, Winship A, Liew SH, et al. The capacity of oocytes for DNA repair[J]. Cell Mol Life Sci, 2018, 75(15):2777-2792. doi: 10.1007/s00018-018-2833-9.
doi: 10.1007/s00018-018-2833-9 pmid: 29748894 |
| [5] |
Wang JJ, Ge W, Zhai QY, et al. Single-cell transcriptome landscape of ovarian cells during primordial follicle assembly in mice[J]. PLoS Biol, 2020, 18(12):e3001025. doi: 10.1371/journal.pbio.3001025.
doi: 10.1371/journal.pbio.3001025 URL |
| [6] |
Sun X, Klinger FG, Liu J, et al. miR-378-3p maintains the size of mouse primordial follicle pool by regulating cell autophagy and apoptosis[J]. Cell Death Dis, 2020, 11(9):737. doi: 10.1038/s41419-020-02965-1.
doi: 10.1038/s41419-020-02965-1 URL |
| [7] |
Wright WD, Shah SS, Heyer WD. Homologous recombination and the repair of DNA double-strand breaks[J]. J Biol Chem, 2018, 293(27):10524-10535. doi: 10.1074/jbc.TM118.000372.
doi: 10.1074/jbc.TM118.000372 URL |
| [8] |
Bhat KP, Krishnamoorthy A, Dungrawala H, et al. RADX Modulates RAD51 Activity to Control Replication Fork Protection[J]. Cell Rep, 2018, 24(3):538-545. doi: 10.1016/j.celrep.2018.06.061.
doi: 10.1016/j.celrep.2018.06.061 URL |
| [9] |
Rickman KA, Noonan RJ, Lach FP, et al. Distinct roles of BRCA2 in replication fork protection in response to hydroxyurea and DNA interstrand cross-links[J]. Genes Dev, 2020, 34(11/12):832-846. doi: 10.1101/gad.336446.120.
doi: 10.1101/gad.336446.120 URL |
| [10] |
Naiman K, Campillo-Funollet E, Watson AT, et al. Replication dynamics of recombination-dependent replication forks[J]. Nat Commun, 2021, 12(1):923. doi: 10.1038/s41467-021-21198-0.
doi: 10.1038/s41467-021-21198-0 pmid: 33568651 |
| [11] |
Carofiglio F, Sleddens-Linkels E, Wassenaar E, et al. Repair of exogenous DNA double-strand breaks promotes chromosome synapsis in SPO11-mutant mouse meiocytes, and is altered in the absence of HORMAD1[J]. DNA Repair (Amst), 2018, 63:25-38. doi: 10.1016/j.dnarep.2018.01.007.
doi: 10.1016/j.dnarep.2018.01.007 URL |
| [12] |
Thomas C, Cavazza T, Schuh M. Aneuploidy in human eggs: contributions of the meiotic spindle[J]. Biochem Soc Trans, 2021, 49(1):107-118. doi: 10.1042/BST20200043.
doi: 10.1042/BST20200043 URL |
| [13] |
Winship AL, Stringer JM, Liew SH, et al. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing[J]. Hum Reprod Update, 2018, 24(2):119-134. doi: 10.1093/humupd/dmy002.
doi: 10.1093/humupd/dmy002 pmid: 29377997 |
| [14] |
Miao Y, Wang P, Xie B, et al. BRCA2 deficiency is a potential driver for human primary ovarian insufficiency[J]. Cell Death Dis, 2019, 10(7):474. doi: 10.1038/s41419-019-1720-0.
doi: 10.1038/s41419-019-1720-0 URL |
| [15] |
ElInati E, Zielinska AP, McCarthy A, et al. The BCL-2 pathway preserves mammalian genome integrity by eliminating recombination-defective oocytes[J]. Nat Commun, 2020, 11(1):2598. doi: 10.1038/s41467-020-16441-z.
doi: 10.1038/s41467-020-16441-z pmid: 32451402 |
| [16] |
Zhao W, Wiese C, Kwon Y, et al. The BRCA Tumor Suppressor Network in Chromosome Damage Repair by Homologous Recombination[J]. Annu Rev Biochem, 2019, 88:221-245. doi: 10.1146/annurev-biochem-013118-111058.
doi: 10.1146/annurev-biochem-013118-111058 URL |
| [17] |
Luo W, Guo T, Li G, et al. Variants in Homologous Recombination Genes EXO1 and RAD51 Related with Premature Ovarian Insufficiency[J]. J Clin Endocrinol Metab, 2020, 105(10):dgaa505. doi: 10.1210/clinem/dgaa505.
doi: 10.1210/clinem/dgaa505 |
| [18] |
Jaillard S, McElreavy K, Robevska G, et al. STAG3 homozygous missense variant causes primary ovarian insufficiency and male non-obstructive azoospermia[J]. Mol Hum Reprod, 2020, 26(9):665-677. doi: 10.1093/molehr/gaaa050.
doi: 10.1093/molehr/gaaa050 pmid: 32634216 |
| [19] |
Heddar A, Beckers D, Fouquet B, et al. A Novel Phenotype Combining Primary Ovarian Insufficiency Growth Retardation and Pilomatricomas With MCM8 Mutation[J]. J Clin Endocrinol Metab, 2020, 105(6):dgaa155. doi: 10.1210/clinem/dgaa155.
doi: 10.1210/clinem/dgaa155 |
| [20] |
Alvarez-Mora MI, Todeschini AL, Caburet S, et al. An exome-wide exploration of cases of primary ovarian insufficiency uncovers novel sequence variants and candidate genes[J]. Clin Genet, 2020, 98(3):293-298. doi: 10.1111/cge.13803.
doi: 10.1111/cge.13803 pmid: 32613604 |
| [21] |
Helbling-Leclerc A, Garcin C, Rosselli F. Beyond DNA repair and chromosome instability-Fanconi anaemia as a cellular senescence-associated syndrome[J]. Cell Death Differ, 2021, 28(4):1159-1173. doi: 10.1038/s41418-021-00764-5.
doi: 10.1038/s41418-021-00764-5 pmid: 33723374 |
| [22] |
Hill RJ, Crossan GP. DNA cross-link repair safeguards genomic stability during premeiotic germ cell development[J]. Nat Genet, 2019, 51(8):1283-1294. doi: 10.1038/s41588-019-0471-2.
doi: 10.1038/s41588-019-0471-2 URL |
| [23] |
Dubois EL, Guitton-Sert L, Béliveau M, et al. A Fanci knockout mouse model reveals common and distinct functions for FANCI and FANCD2[J]. Nucleic Acids Res, 2019, 47(14):7532-7547. doi: 10.1093/nar/gkz514.
doi: 10.1093/nar/gkz514 URL |
| [24] |
Tsui V, Crismani W. The Fanconi Anemia Pathway and Fertility[J]. Trends Genet, 2019, 35(3):199-214. doi: 10.1016/j.tig.2018.12.007.
doi: 10.1016/j.tig.2018.12.007 URL |
| [25] |
Yang X, Zhang X, Jiao J, et al. Rare variants in FANCA induce premature ovarian insufficiency[J]. Hum Genet, 2019, 138(11/12):1227-1236. doi: 10.1007/s00439-019-02059-9.
doi: 10.1007/s00439-019-02059-9 URL |
| [26] |
Kolinjivadi AM, Crismani W, Ngeow J. Emerging functions of Fanconi anemia genes in replication fork protection pathways[J]. Hum Mol Genet, 2020, 29(R2):R158-R164. doi: 10.1093/hmg/ddaa087.
doi: 10.1093/hmg/ddaa087 pmid: 32420592 |
| [27] |
Lowran K, Campbell L, Popp P, et al. Assembly of a G-Quadruplex Repair Complex by the FANCJ DNA Helicase and the REV1 Polymerase[J]. Genes (Basel), 2019, 11(1):5. doi: 10.3390/genes11010005.
doi: 10.3390/genes11010005 URL |
| [28] |
Panday A, Willis NA, Elango R, et al. FANCM regulates repair pathway choice at stalled replication forks[J]. Mol Cell, 2021, 81(11):2428-2444. doi: 10.1016/j.molcel.2021.03.044.
doi: 10.1016/j.molcel.2021.03.044 URL |
| [29] |
McNairn AJ, Chuang CH, Bloom JC, et al. Female-biased embryonic death from inflammation induced by genomic instability[J]. Nature, 2019, 567(7746):105-108. doi: 10.1038/s41586-019-0936-6.
doi: 10.1038/s41586-019-0936-6 URL |
| [30] |
Jaillard S, Bell K, Akloul L, et al. New insights into the genetic basis of premature ovarian insufficiency: Novel causative variants and candidate genes revealed by genomic sequencing[J]. Maturitas, 2020, 141:9-19. doi: 10.1016/j.maturitas.2020.06.004.
doi: 10.1016/j.maturitas.2020.06.004 URL |
| [31] |
Caburet S, Heddar A, Dardillac E, et al. Homozygous hypomorphic BRCA2 variant in primary ovarian insufficiency without cancer or Fanconi anaemia trait[J]. J Med Genet, 2020 Jun 1:jmedgenet-2019-106672. doi: 10.1136/jmedgenet-2019-106672.
doi: 10.1136/jmedgenet-2019-106672 |
| [32] |
Hatano Y, Tamada M, Matsuo M, et al. Molecular Trajectory of BRCA1 and BRCA2 Mutations[J]. Front Oncol, 2020, 10:361. doi: 10.3389/fonc.2020.00361.
doi: 10.3389/fonc.2020.00361 URL |
| [33] |
Alter BP, Best AF. Frequency of heterozygous germline pathogenic variants in genes for Fanconi anemia in patients with non-BRCA1/BRCA2 breast cancer: a meta-analysis[J]. Breast Cancer Res Treat, 2020, 182(2):465-476. doi: 10.1007/s10549-020-05710-6.
doi: 10.1007/s10549-020-05710-6 URL |
| [34] |
Hill P, Leitch HG, Requena CE, et al. Epigenetic reprogramming enables the transition from primordial germ cell to gonocyte[J]. Nature, 2018, 555(7696):392-396. doi: 10.1038/nature25964.
doi: 10.1038/nature25964 URL |
| [35] |
Singh V, Kumar Mohanty S, Verma P, et al. XRCC1 deficiency correlates with increased DNA damage and male infertility[J]. Mutat Res Genet Toxicol Environ Mutagen, 2019, 839:1-8. doi: 10.1016/j.mrgentox.2019.01.004.
doi: 10.1016/j.mrgentox.2019.01.004 URL |
| [36] |
Toledo M, Sun X, Brieño-Enríquez MA, et al. A mutation in the endonuclease domain of mouse MLH3 reveals novel roles for MutLγ during crossover formation in meiotic prophase I[J]. PLoS Genet, 2019, 15(6):e1008177. doi: 10.1371/journal.pgen.1008177.
doi: 10.1371/journal.pgen.1008177 URL |
| [37] |
Milano CR, Holloway JK, Zhang Y, et al. Mutation of the ATPase Domain of MutS Homolog-5 (MSH5) Reveals a Requirement for a Functional MutSγ Complex for All Crossovers in Mammalian Meiosis[J]. G3 (Bethesda), 2019, 9(6):1839-1850. doi: 10.1534/g3.119.400074.
doi: 10.1534/g3.119.400074 URL |
| [38] |
Akbari A, Padidar K, Salehi N, et al. Rare missense variant in MSH4 associated with primary gonadal failure in both 46, XX and 46, XY individuals[J]. Hum Reprod, 2021, 36(4):1134-1145. doi: 10.1093/humrep/deaa362.
doi: 10.1093/humrep/deaa362 URL |
| [39] |
Cui T, Srivastava AK, Han C, et al. DDB2 represses ovarian cancer cell dedifferentiation by suppressing ALDH1A1[J]. Cell Death Dis, 2018, 9(5):561. doi: 10.1038/s41419-018-0585-y.
doi: 10.1038/s41419-018-0585-y URL |
| [40] |
Das S, Naher L, Aka TD, et al. The ECCR1 rs11615, ERCC4 rs2276466, XPC rs2228000 and XPC rs2228001 polymorphisms increase the cervical cancer risk and aggressiveness in the Bangladeshi population[J]. Heliyon, 2021, 7(1):e05919. doi: 10.1016/j.heliyon.2021.e05919.
doi: 10.1016/j.heliyon.2021.e05919 URL |
| [41] |
Katari S, Aarabi M, Kintigh A, et al. Chromosomal instability in women with primary ovarian insufficiency[J]. Hum Reprod, 2018, 33(3):531-538. doi: 10.1093/humrep/dey012.
doi: 10.1093/humrep/dey012 URL |
| [1] | 肖楠, 李永程, 姚义鸣, 孙红文, 姚汝强, 陈泳君, 殷宇辰, 罗海宁. 卵巢微环境内邻苯二甲酸酯暴露与炎性因子水平的关系[J]. 国际生殖健康/计划生育杂志, 2024, 43(5): 353-360. |
| [2] | 王冬雪, 包莉莉, 刘珊, 杨波. 改良灵活拮抗剂方案对卵巢功能正常女性COH结局的影响[J]. 国际生殖健康/计划生育杂志, 2024, 43(3): 185-189. |
| [3] | 梁越, 董杰, 肖西峰, 王晓红. miR-202在生殖调控中的研究进展[J]. 国际生殖健康/计划生育杂志, 2024, 43(3): 228-233. |
| [4] | 高朝阳, 章宁晴, 陈琼华, 吴荣锋. 环状RNA在子宫内膜异位症不孕患者卵泡颗粒细胞中的作用[J]. 国际生殖健康/计划生育杂志, 2024, 43(3): 243-248. |
| [5] | 曹媛媛, 贾赞慧, 张春苗. ZP1基因突变在空卵泡综合征中的研究进展[J]. 国际生殖健康/计划生育杂志, 2024, 43(2): 127-131. |
| [6] | 甄佳, 赵紫渊, 王子璐, 师伟, 徐丽. 多囊卵巢综合征病理机制中的颗粒细胞自噬[J]. 国际生殖健康/计划生育杂志, 2024, 43(2): 150-154. |
| [7] | 吴静, 刘聪, 谢青贞. 微塑料暴露对雌性及其子代健康的影响[J]. 国际生殖健康/计划生育杂志, 2024, 43(2): 155-158. |
| [8] | 李文雅, 张巧利, 杨晓葵. 内质网应激在多囊卵巢综合征中的研究进展[J]. 国际生殖健康/计划生育杂志, 2024, 43(1): 53-57. |
| [9] | 李彩华, 郭培培, 姜小花, 方有燕, 周平, 魏兆莲. 卵泡期高孕激素状态下促排卵方案的应用进展[J]. 国际生殖健康/计划生育杂志, 2024, 43(1): 68-73. |
| [10] | 向春蓉, 邓志敏, 代芳芳, 程艳香. 间充质干细胞及其外泌体治疗早发性卵巢功能不全的临床研究及其进展[J]. 国际生殖健康/计划生育杂志, 2023, 42(6): 492-497. |
| [11] | 叶明珠, 郑洁, 李杰芃, 许莉欣. 医源性卵巢储备功能减退患者的卵母细胞冷冻生育力保存应用[J]. 国际生殖健康/计划生育杂志, 2023, 42(6): 498-502. |
| [12] | 柳絮, 杨爱军, 李泽武, 石城, 刘利君, 孔潇丽, 王靖雯. 富血小板血浆改善卵巢储备功能的相关机制[J]. 国际生殖健康/计划生育杂志, 2023, 42(4): 329-333. |
| [13] | 陈楸妍, 鲁南, 刘嘉茵. 生长激素预处理在前次IVF/ICSI失败非DOR患者中的临床应用[J]. 国际生殖健康/计划生育杂志, 2023, 42(3): 184-188. |
| [14] | 李延, 胡方方, 陈欢欢, 张磊, 张翠莲, 梁琳琳. 窦前卵泡体外三维培养系统研究进展[J]. 国际生殖健康/计划生育杂志, 2023, 42(3): 221-225. |
| [15] | 杨志娟, 姚婷, 侯海燕. 线粒体自噬与卵巢功能[J]. 国际生殖健康/计划生育杂志, 2023, 42(3): 240-244. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||